Last year when I was at home with my young infant daughter during the holidays I watched Lucinda Scala Quinn (on the TV show Mad Hungry) cure a salmon. A properly cured salmon does not need to be cooked because the brine mixture removes any dangerous substances.
This brought back memories of my visit to the Dead Sea Scrolls exhibit in Seattle a few years back. I'm fascinated that the high salt content of the Dead Sea created a sterile environment in which the ancient texts of the Old Testament were discovered. The same chemical that cures a salmon for my dinner created a preservative in which historical documents survived for a record amount of time.
Initially, neither the salmon nor the Dead Sea Scrolls exhibit seemed all that relevant to my chemistry teaching career. While one is delicious to eat and the other a seeming wonder of the world, the two seemed unconnected to each other and to my academic major .
Except that they both involve preparation and preservation with salt. And in the nitty gritty of it all, this is really exciting.
What people do not realize is that the word "salt" is a completely different word in science than it is in the kitchen. In the kitchen "salt" is sodium chloride only. You can say "salt" in cooking and everybody knows exactly what you are talking about. In science, the word "salt" encompasses any compound that contains an ionically bonded species, whether it be a metal/nonmetal combination or some other variety of cation/anion combination. Any neutralization reaction between an acid and a hydroxide base yields some kind of salt (plus water). The word salt is such a general term in science that, by itself, just refers to something that forms ions in solution.
Salts played a pivotal role in the historical development of chemistry as a discipline. The medieval alchemist Paracelsus named salt as a third principle of nature. However, he treated it more as a category of nature and his work became known throughout Europe in the middle of the 1700s. As the chemical behavior of salts became better understood, there was a significant increase in the number of salts known and this resulted in a need for a classification system. Classification as a method of understanding became a defining characteristic of science in general and of chemistry as a discipline.
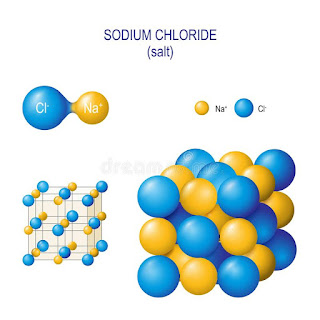
Although salts historically include the many, many compounds of the first classification scheme in chemistry, we are exclusively referring to NaCl in the kitchen.
In food preservation and flavoring, salt (NaCl) is used to create a diffusion of water from a lower salt concentration to a higher salt concentration. When this happens we say osmosis occurs. This brings me back to previous posts about polarity, shape and solubility. What is it about salt that causes this to occur? It is ionic bonding.
One common misconception about salt preservation is that salty foods around the house are more likely to be free of bacteria. This is false based on the percentage salt needed to create this effect. The food must be at least 10% salt to kill bacteria (possibly closer to 20%). Most salty foods do not have more than one or two percent salt.
As far as salt's mechanism for flavoring, the salt loosens the tight protein coils so that more water is trapped in the fiber of the meat/fish. This makes the fish juicier.
All of this inspires me to use my newly purchased turkey brine from Williams Sonoma to enhance the Thanksgiving Day meal at our house. Although I will not be adding enough salt to remove all bacteria from my poultry (and thus I will cook it), I will allow more water to penetrate the meat making it juicier and a more delectable meal for all.
And I will have new anecdotal and historical information to share with my holiday guests about salt preservation and flavoring. Salt not only played a critical role in defining science as a discipline separate from philosophy or religion but it preserved historical documents (Dead Sea Scrolls), and flavors our everyday meals. I am most thankful for salt this holiday season.
Figure: Sodium chloride crystal structure